Chiral TLC Plates
Reversed-phase nano-silica impregnated with Cu(II) ions
and a chiral selector (proline derivative)
Chiral TLC plates enantiomer separation is based on reversed phase ligand exchange, i.e. formation of tertiary mixed-ligand complexes with the Cu(II) ions. These differences in the stability of the diastereomeric complexes produce the chromatographic separation.
These plates can be used effectively for scouting a suitable chiral chromatography separation mode for further analysis within column chromatography or HPLC applications.
Chiral TLC Plates Specifications:
- Silica gel impregnated with Cu(II) ions and a chiral selector (proline derivative)
- pH stability 2 – 8
- Irregular shape, fully porous particles
- 250μm layer thickness
-
Glass plates
-
Fluorescent indicator
Recommended Applications:
- Amino acids, Dipeptides, Enantiomers, Lactones, N-formylamino acids, N-methylamino acids, Thiazolidine derivatives, α-alkylamino acids, α-hydroxycarboxylic acids
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 3415126 | Silica Chiral TLC Plates, w/UV254, glass backed, 250um, 20x20cm 25/pk | |
| 3415136 | Silica Chiral TLC Plates, w/UV254, glass backed, 250um, 10x20cm 25/pk |  Buy now $822.25 Buy now $822.25 |
| 3415131 | Silica Chiral TLC Plates, w/UV254, glass backed, 250um, 10x20cm 4/pk |  Buy now $169.05 Buy now $169.05 |
| 3415147 | Silica Chiral TLC Plates, w/UV254, glass backed, 250um, 5x20cm 50/pk |  Buy now $914.25 Buy now $914.25 |
| 3415156 | Silica Chiral TLC Plates, w/UV254, glass backed, 250um, 10x10cm 25/pk |  Buy now $678.50 Buy now $678.50 |
Chromatographic conditions for use of Chiral TLC Plates
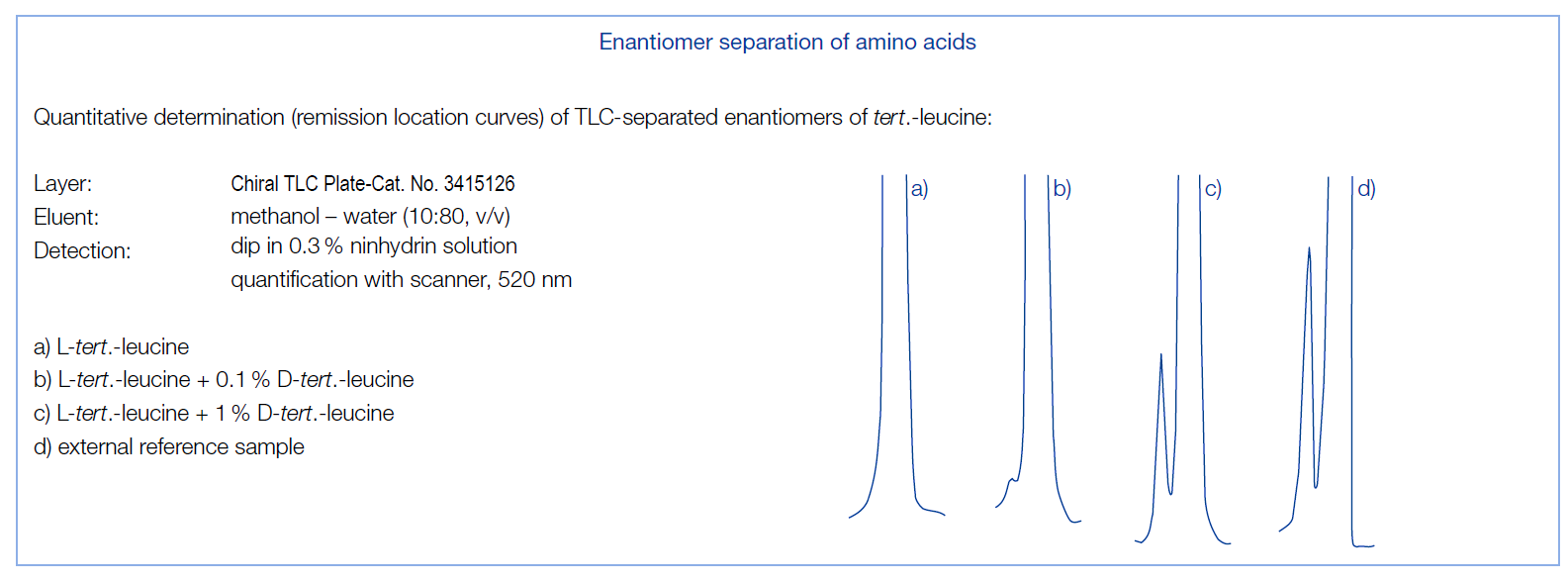
No circumstantial dippings in different solvents or solutions are necessary. For amino acid derivatives most of the separations achieved so far have been run with a mixture of methanol-water-acetonitrile in the ratio 50:50:200 v/v/v (eluent A, developing time about 30 minutes) or 50:50:30 v/v/v (eluent B, developing time about 60 minutes). By changing the amount of acetonitrile in the developing solvent you can influence efficiency and separation time. Lowering the acetonitrile concentration rapidly increases separation times. U. A. Th. Brinkman et al.1) investigated the influence of the composition of different binary mixtures of acetonitrile and water on the separation of different amino acid racemates (see fig. below).
Though the above mentioned solvent mixtures are useful to separate many substances, in special cases other eluents may be required 2). Thus leucine can be separated in a mixture of methanol-water (10:80 v/v, developing time about 90 min); alanine and serine can be resolved with acetone-methanol-water (10:2:2 v/v/v, developing time ~ 50 min); N-carbamyl-tryptophan is separated with 1 mM cupric acetate solution containing 5% methanol (pH 5.8) 3) and enantiomeric α-hydroxycarboxylic acids could be resolved using dichloromethane-methanol (45: 5 v/v, developing time about 20 min) 2), 4).


