TLC for Flash Chromatography
Use TLC to Evaluate the Most Efficient Flash Run Conditions
Thin Layer Chromatography (TLC) is an excellent first-step solution for the development of a selective and reproducible method in Flash chromatography. TLC permits the user to evaluate several eluents and adsorbent/column combinations quickly and without major cost to find the optimal conditions to maximize the efficiency and separation of the flash run.
Use TLC to save time, save solvent, and save samples before using column chromatography.

Thin Layer Chromatography (TLC) will help you determine:
- What solvent system would present the best separation
- What adsorbent would provide the greatest retention of character
- What size column and sample load would be optimal
- Approximate column volumes of solvent to elute the samples of interest
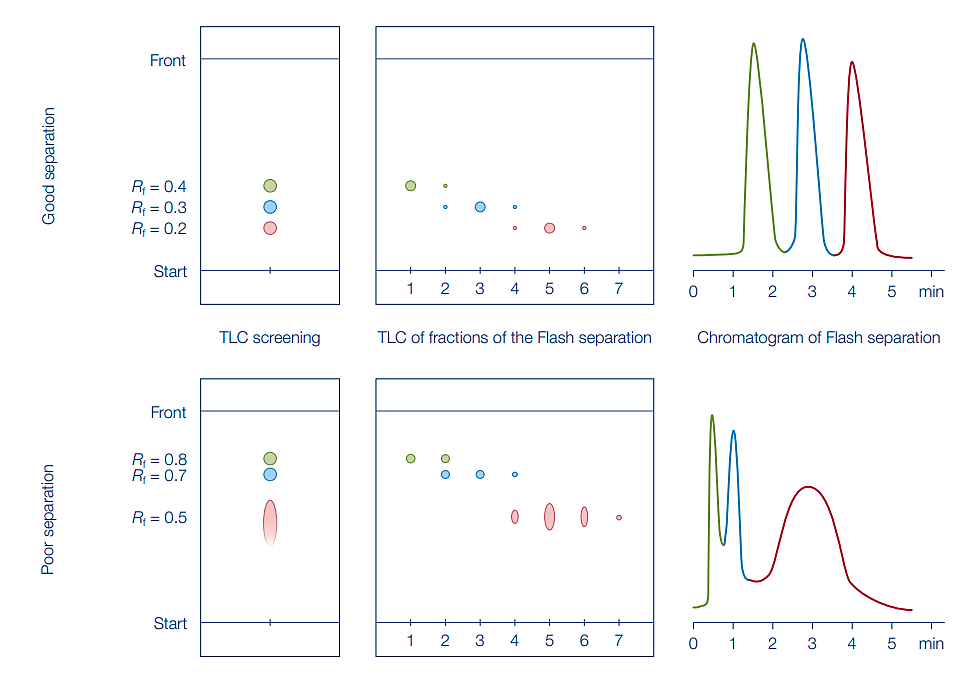
How does it work?
First, run a TLC screen using our normal phase silica gel plates. If separation isn’t able to be observed, you can also use bonded phase HPTLC plates to provide an alternative to the traditional normal phase TLC.
Determine the retention factor or Rf value for each spotted analyte within your mixture. The Rf value is calculated using the following formula:
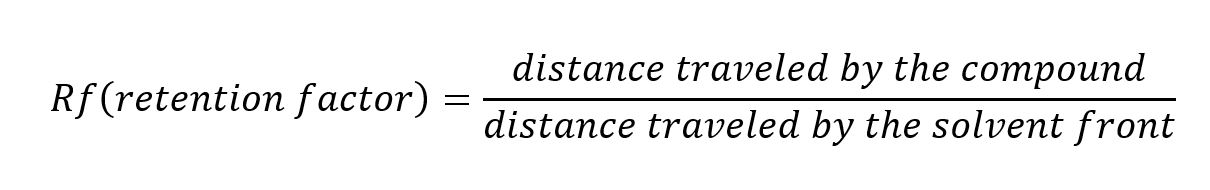
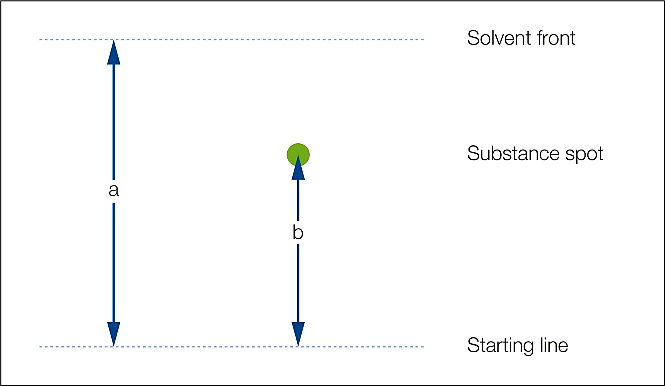
A suitable solvent system should produce an Rf range between 0.13 – 0.4, with an Rf between compounds of at least 0.1.
Conversion of Rf to Column Volumes (CV) for Flash Chromatography
Since TLC uses the retention factor or Rf, we need to convert the TLC Rf to column volume or CV. A Column Volume is the amount of solvent that is contained in the interstitial space between silica particles and the empty space in the pores of the silica. CV is a more appropriate measurement to be used for flash separations as it can be used to calculate the amount of solvent required to elute a compound of interest.
To convert from Rf to CV:
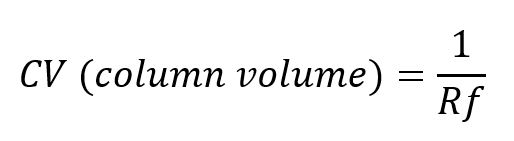
The greater the difference in CVs, the more sample that can be loaded on the flash cartridge, while still maintaining compound purity.
An optimal CV range for flash is recommended to be a CV of between 1.8 to 4.5. Smaller CV values can result in in lower purity of the desired compounds. Higher CV values can lead to very long retention times and higher solvent usage.


