Silica Gel Bonded Phase (Functionalized)
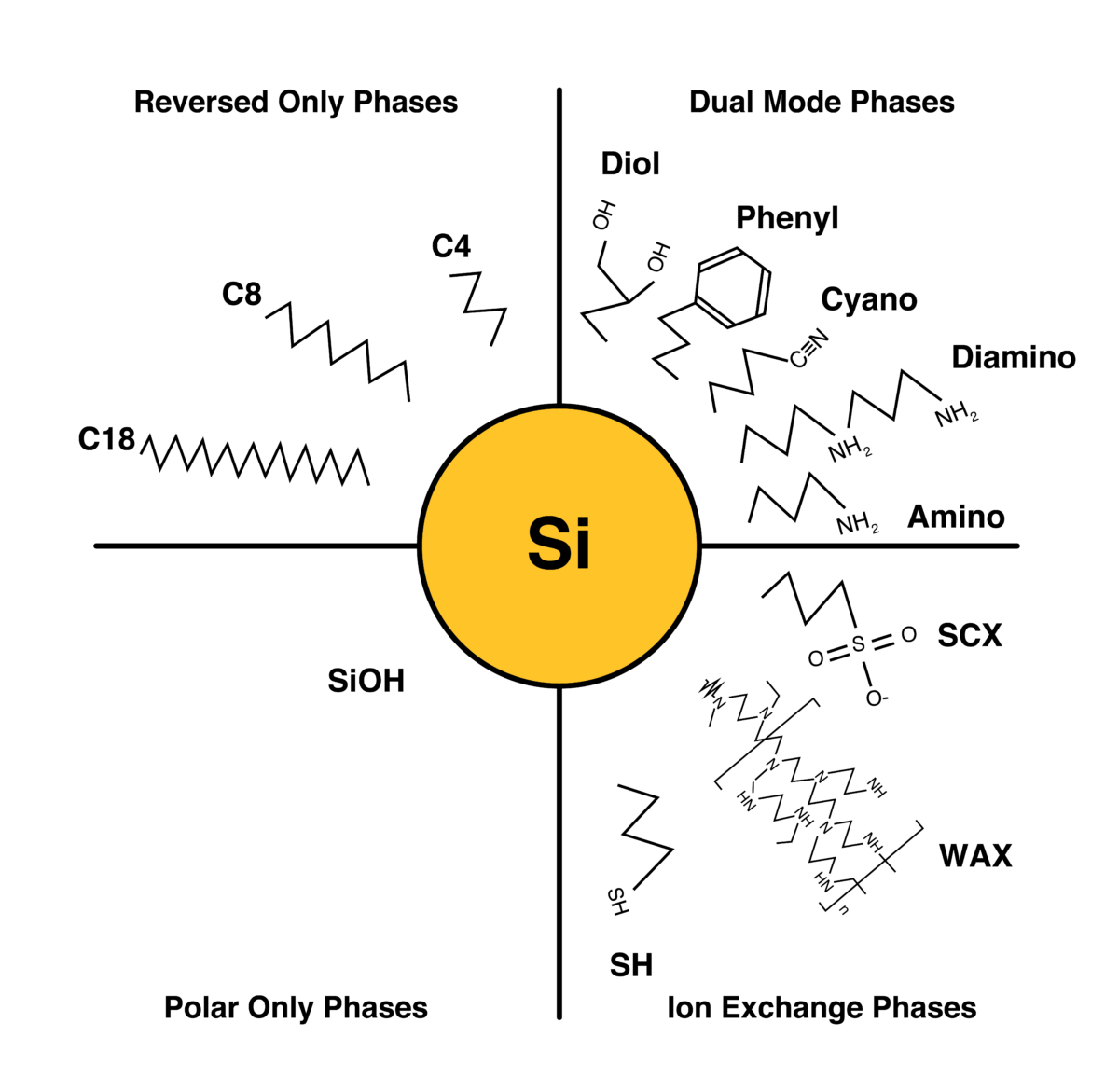
Today’s chemists have access to silica gel bonded phase options that have been developed over the course of many years as surface bonding chemistries progressed.
The various bonded phases are available on our high purity spherical silica gels. The fully porous particles range from 1.8um to 200um, which allows the end-user to go from analytical to preparative to process scale, without any significant variation in the stationary phase. Scale-up with ease by eliminating any variables caused by change in particle shape, bonding chemistry, or manufacturer method.
1 – Reversed Phase Bonding using Monochloro-Silanes
Standard Monofunctional RP-Bonding for C18, C8, C4, etc.
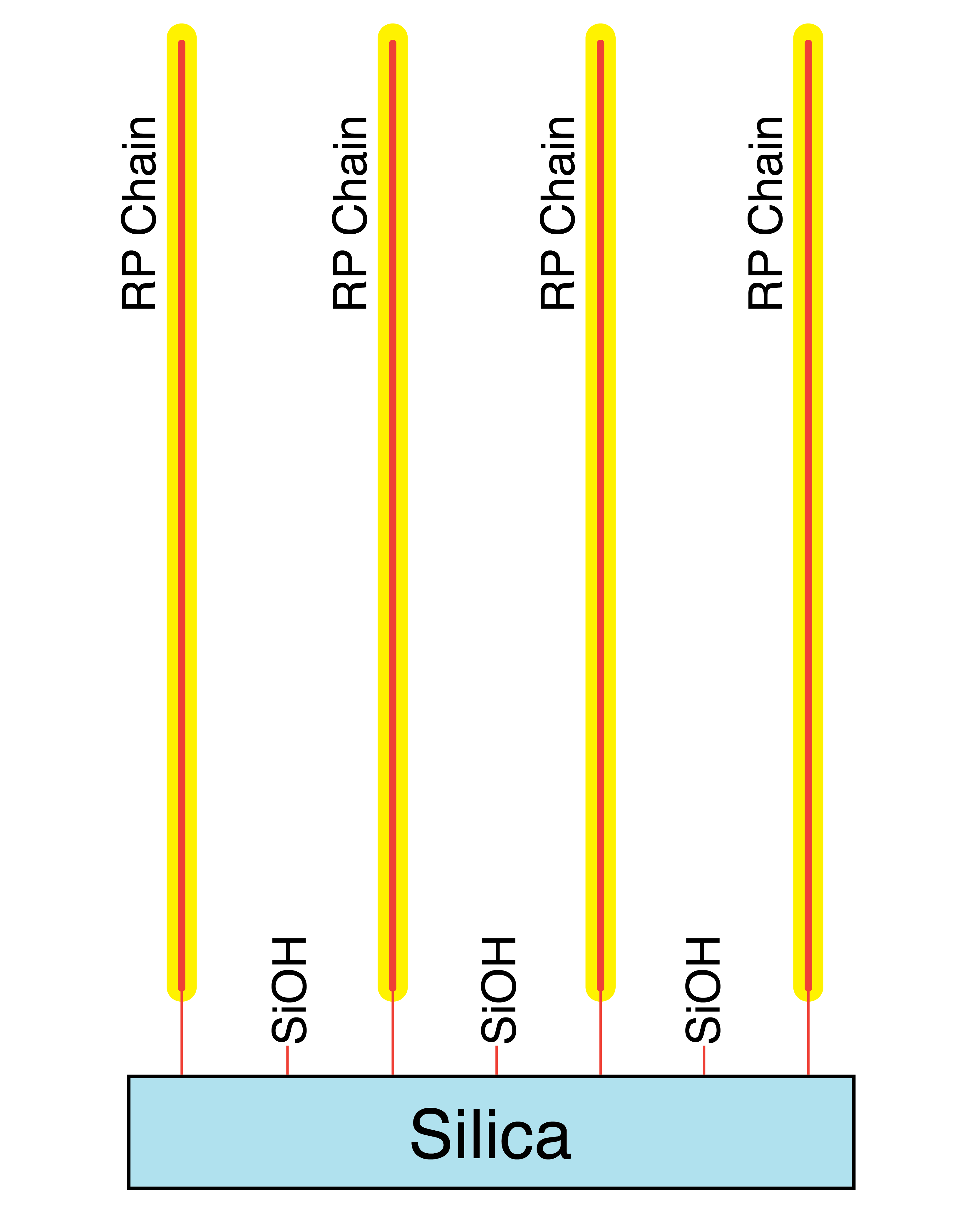
Mixed Mode RP-Bonding for C18, C8, and C4 (Aqueous I and Aqueous II Phases)
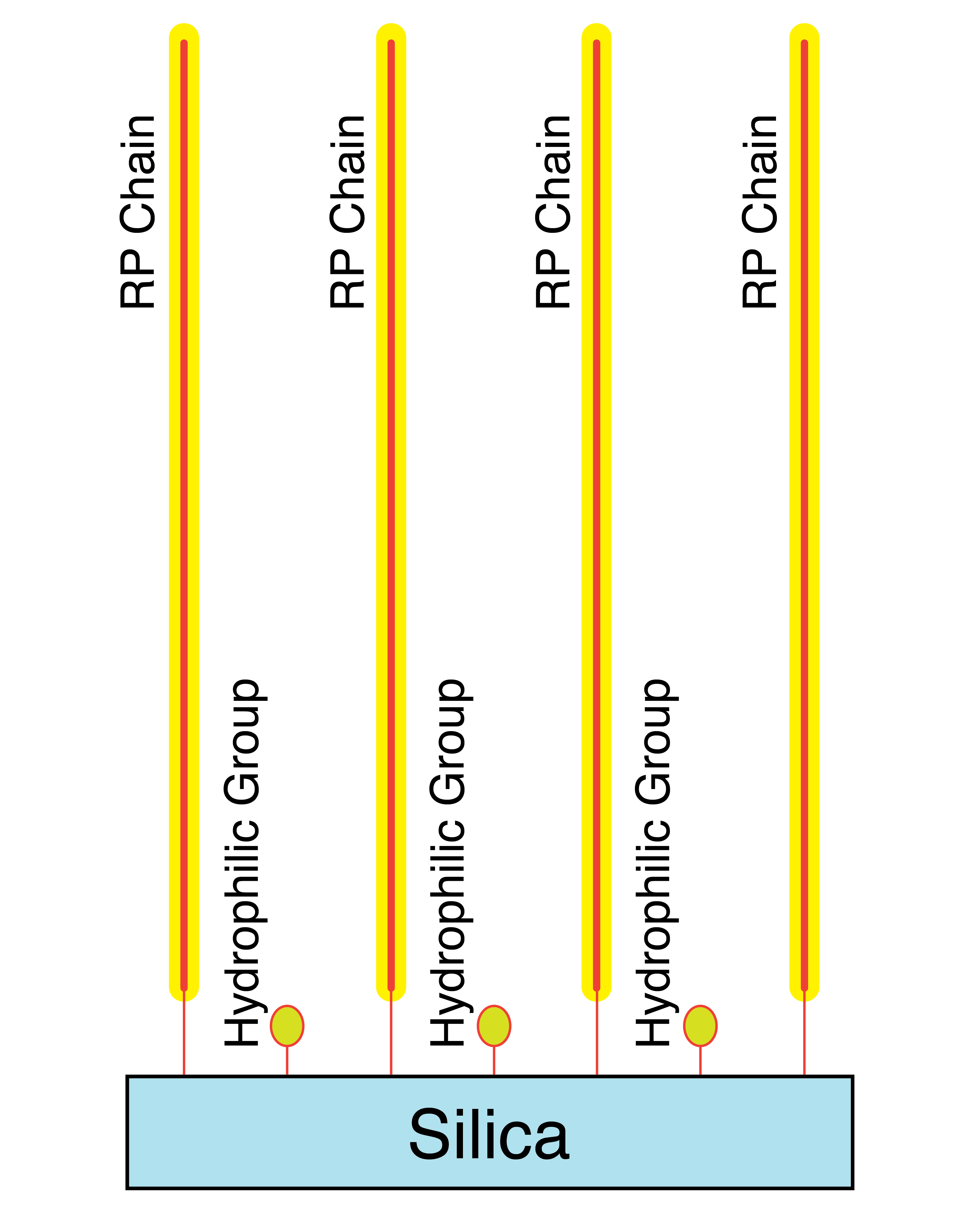
Mixed Mode Endcapping
When the endcapper leaves the residual silanols with only alkyl groups, the overall effect of the primary and secondary silane reactions yields a highly hydrophobic surface coating. This is good for highly hydrophobic analytes when the organic part of the mobile phase is very high.
When the endcapper leaves the residual silanols with hydrophilic groups such as alcohols or amides, the overall effect of the primary and secondary silane reactions yields a less hydrophobic surface coating that is more compatible with high water content mobile phases necessary for highly polar analytes
Endcapping RP-Bonding for C18, C8, C4, etc.
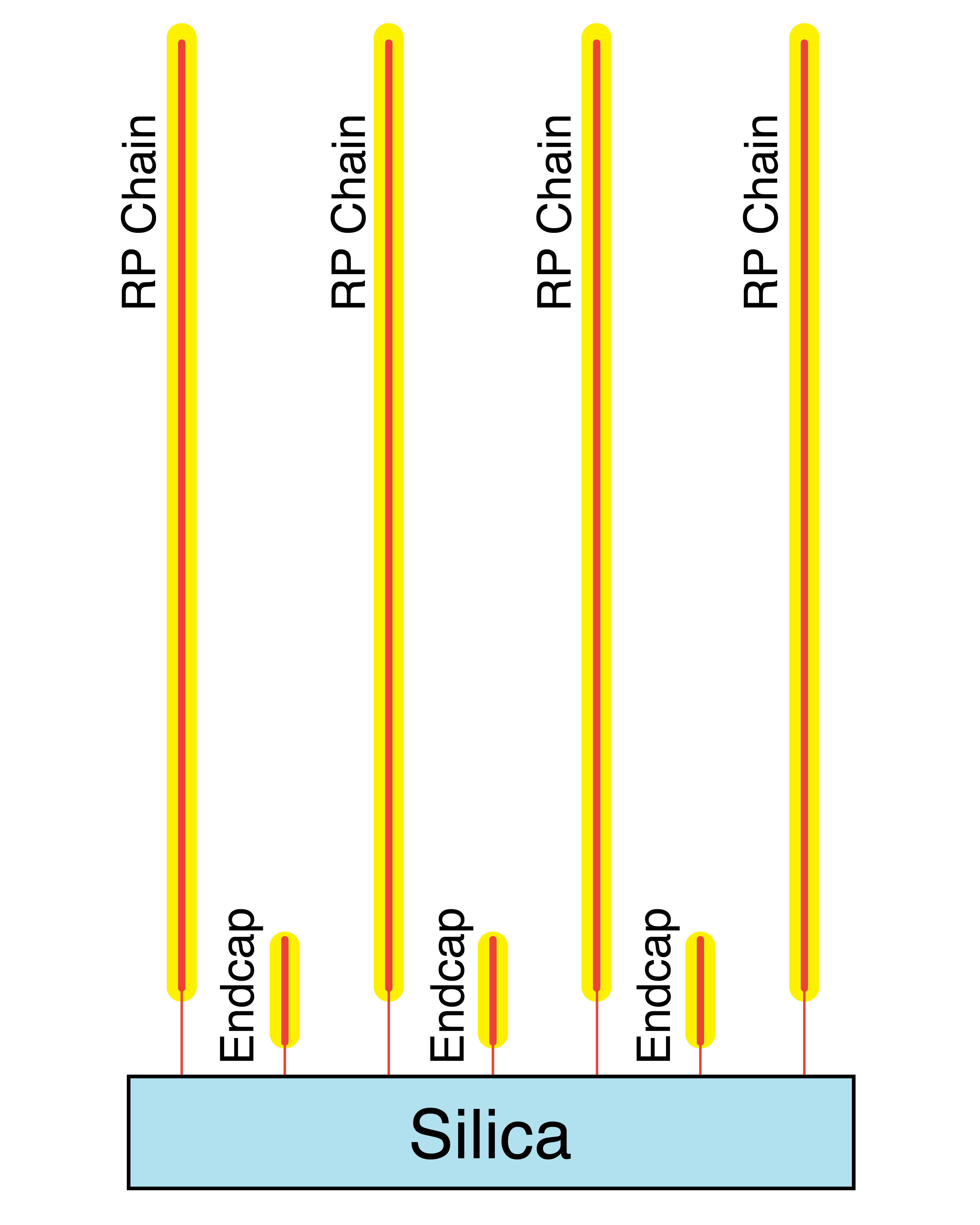
What is endcapping?
Endcapping is the reaction of accessible silanol groups in a bonded stationary phase by a second smaller silane reagent. End-capped stationary phases have significantly lower residual silanol group activity compared to non-endcapped stationary phases.
Non Endcapping (“NE”)
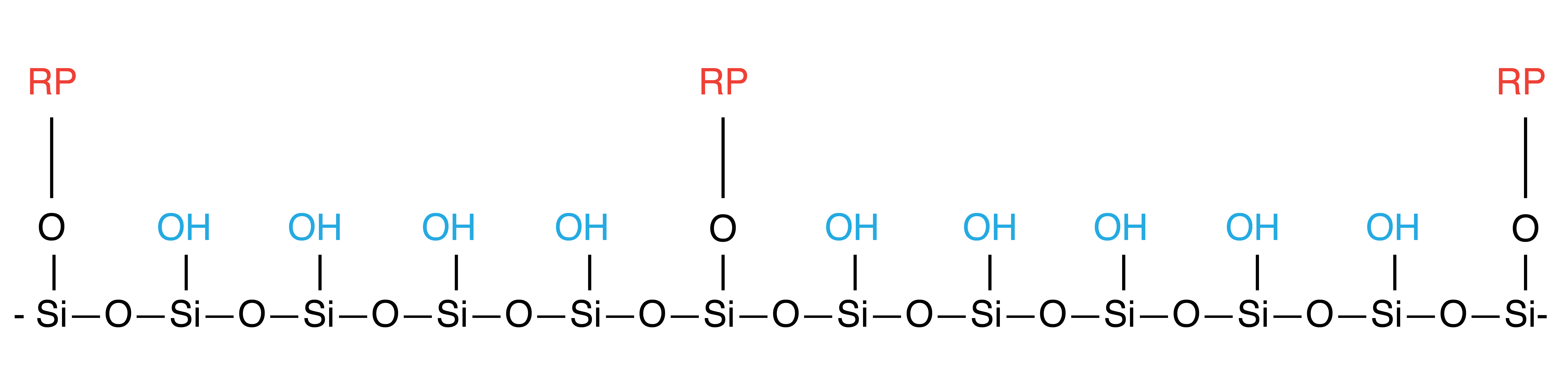
After the initial application of the first silane reagent to the surface of the silica gel, there still remains some "residual" silanol groups that have not reacted with the first silane reaction.
The somewhat acidic SiOH groups can interact with the sample, especially basic analytes, so it is preferable to reduce the number of residual silanol groups. A second reaction is performed with a smaller (shorter length) silane reagent. This is called endcapping. The smaller size is necessary to penetrate the first coating – which is much bulkier – and thereby reacts with the previously unreacted free (residual) silanols on the silica surface.
Liquid Endcapping ("LE")
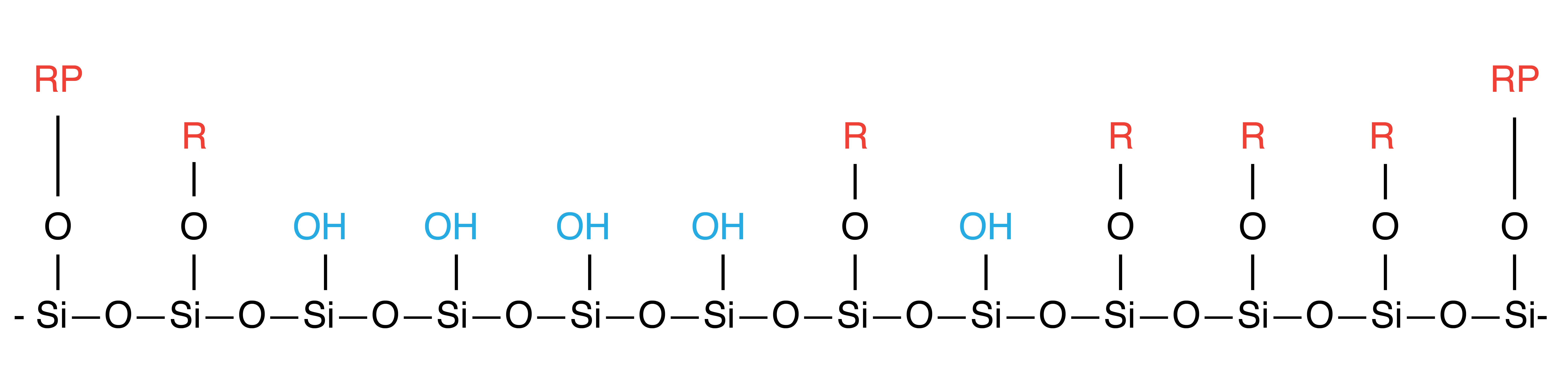
The two methods of accomplishing this use either liquid phase silanes or gas phase silanes. The liquid phase endcapping method (max. 40%) is not as thorough as the gas phase endcapping method (~99%) in covering the available silanol groups on the surface of the silica gel, however for most chromatography techniques (e.g. flash chromatography columns) it is more than satisfactory. For applications where a higher endcapping is desired (e.g. HPLC columns), the gas phase endcapping method is used and will yield a highly inert, hydrophobic surface.
Gas Endcapping (“HE”)
2 – Reversed Phase Bonding using Trichlorosilanes
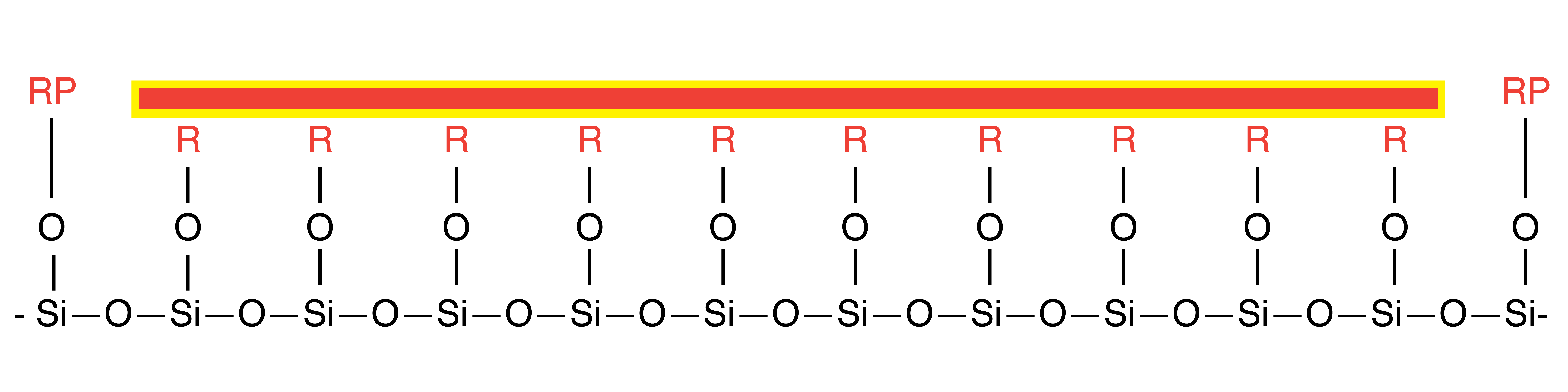
Trifunctional RP-Bonding relies on a trifunctional silane, in this case, trichlorosilane, to bond with both the surface of the silica gel and other trichlorosilanes. Because of these condensation reactions, a C18 web can be created that protects the surface silanols from interacting with the compounds being separated. Usually, three chains per silanol are the maximum, but there can be more.
Standard Trifunctional RP-Bonding for C18, C8, C4, Phenyl, etc.
3 – Bonded Silica for Normal Phase
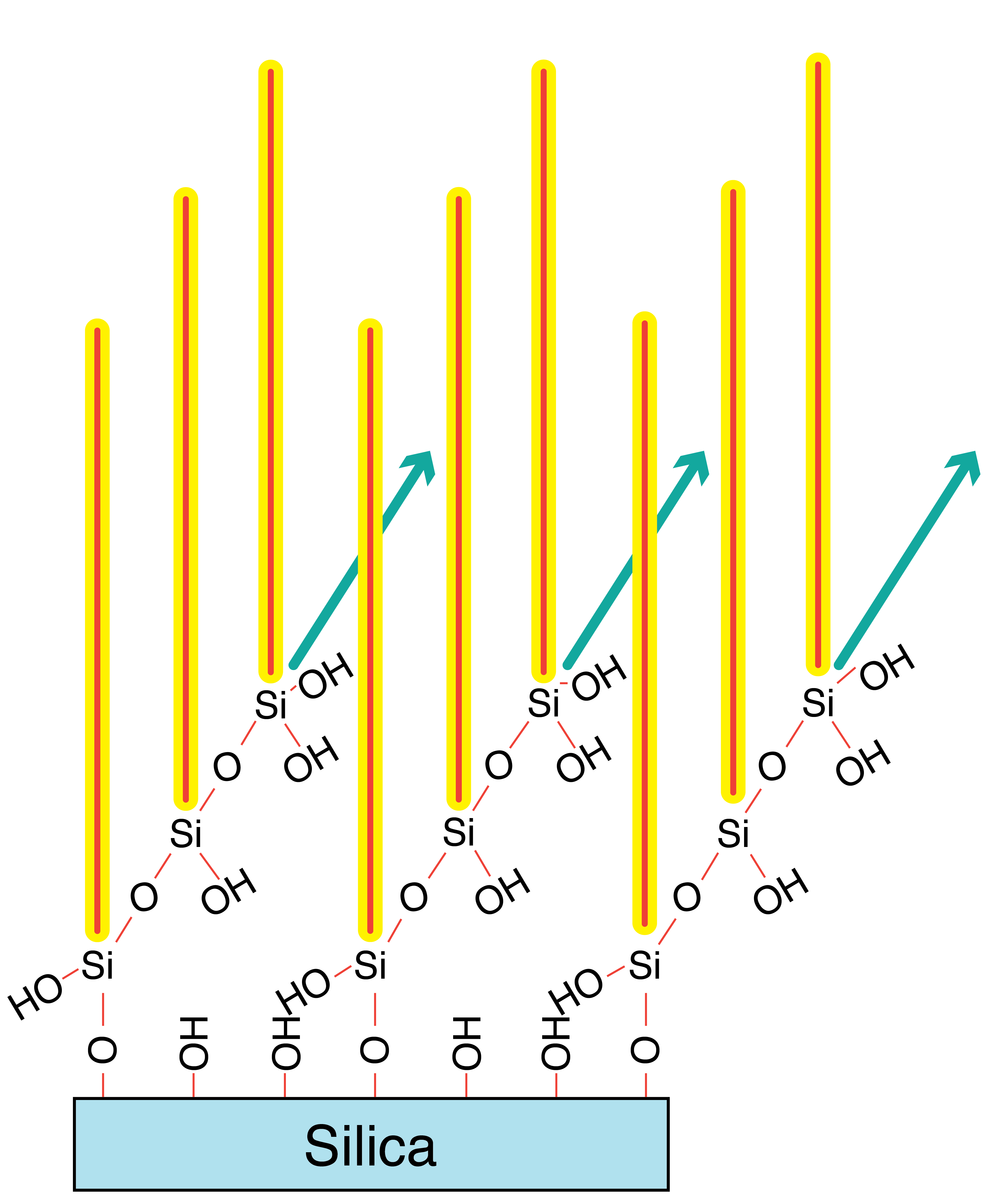
When considering Normal Phase chromatography techniques, plain silica gel is the optimal stationary phase due to its high surface area (500-600 m²/g). Additionally, a 70Å pore diameter works well for most analytes, especially those having a molecular weight of less than 1,000 Daltons. Higher molecular weight analytes require larger pore diameters (>100Å) for entrance into pores as well as optimal interaction with the surface chemistry.
Water also plays an important role in that an increase of the water on the surface will shorten elution times by deadening the silica surface.
Unmodified silica gel offers the following advantages:
- Acidic surface pH
- High loading capacity
- Ease of solvent evaporation from products
Difficulty arises however when certain organic additives (such as triethylamine (TEA)) are added to the mobile phase.
- These additives are hard (virtually impossible) to completely remove
- Note that the use of strong acids or bases may be harmful to some target analytes
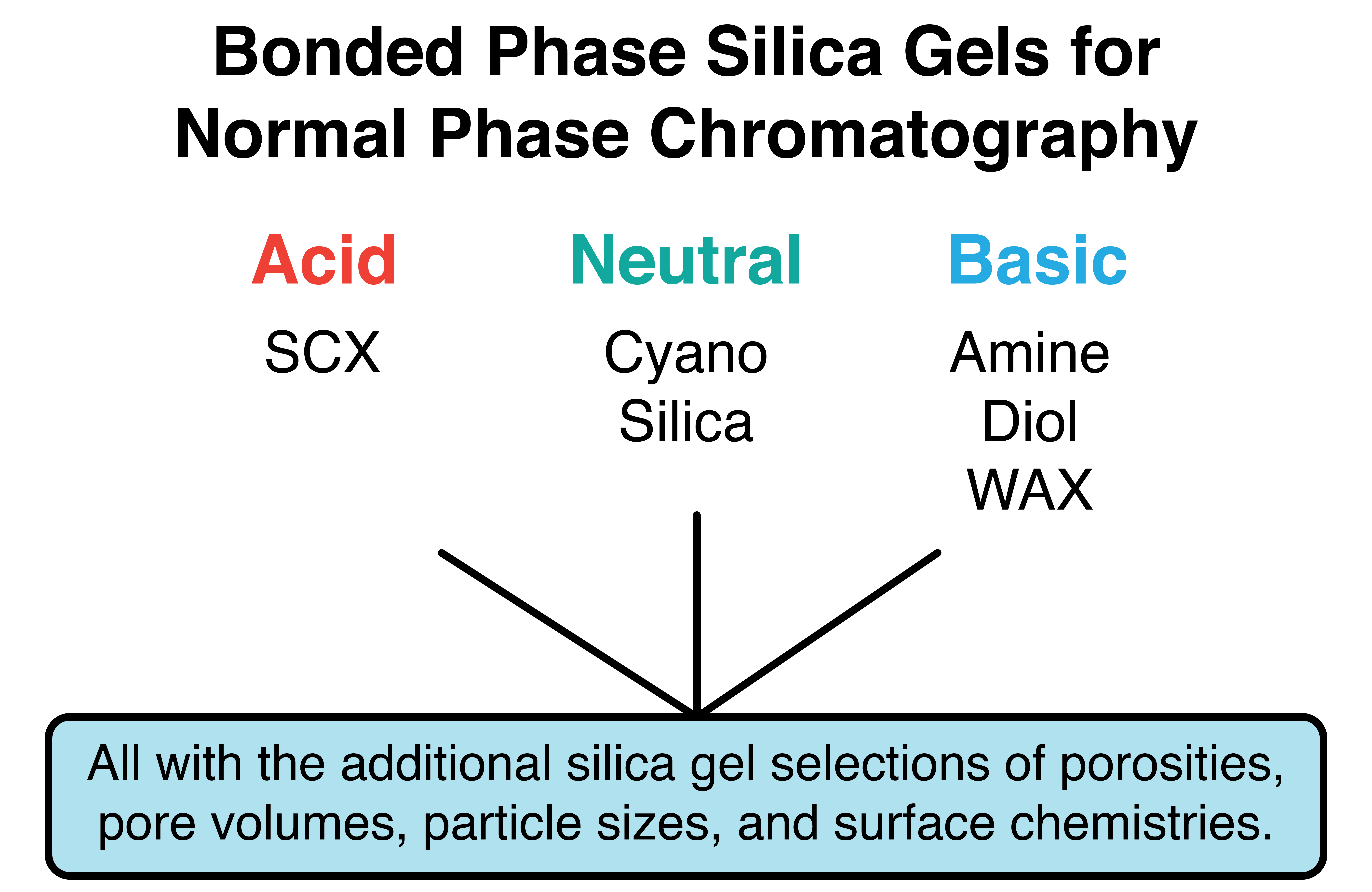
In cases like this, a modified silica gel would serve well. These modified silica gels will increase selectivity for acid, neutral, and basic compound separations.
| Unbonded Silica for Normal Phase | Bonded Silicas for Normal Phase |
|---|---|
| Advantages | Advantages |
| High loading capacity | Generally, more stable |
| More likely to use solvents that evaporate easily | Highly reproducible |
| Can be used for both normal phase and reversed phase | |
| Better selectivity for acidic, neutral, and basic conditions | |
| Limitations | Limitations |
| Hard to remove mobile phase additives | Higher cost |
| Strong acids and bases can affect chromatographic separations | Bonding can be destroyed in extreme conditions |
4 – Silica Gel Bonded Phase for Reversed Phase
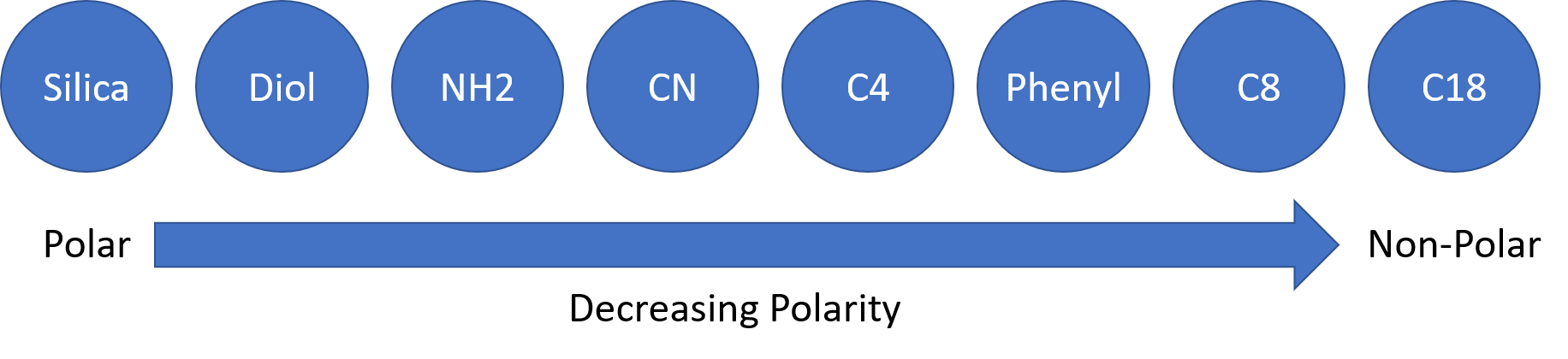
When considering Reversed Phase chromatography techniques, deciding which bonded phase to use as your stationary phase can be difficult. If the compound is more polar or non-polar, this information can be used to select the appropriate stationary phase based on the polar or non-polar nature of the stationary phase.
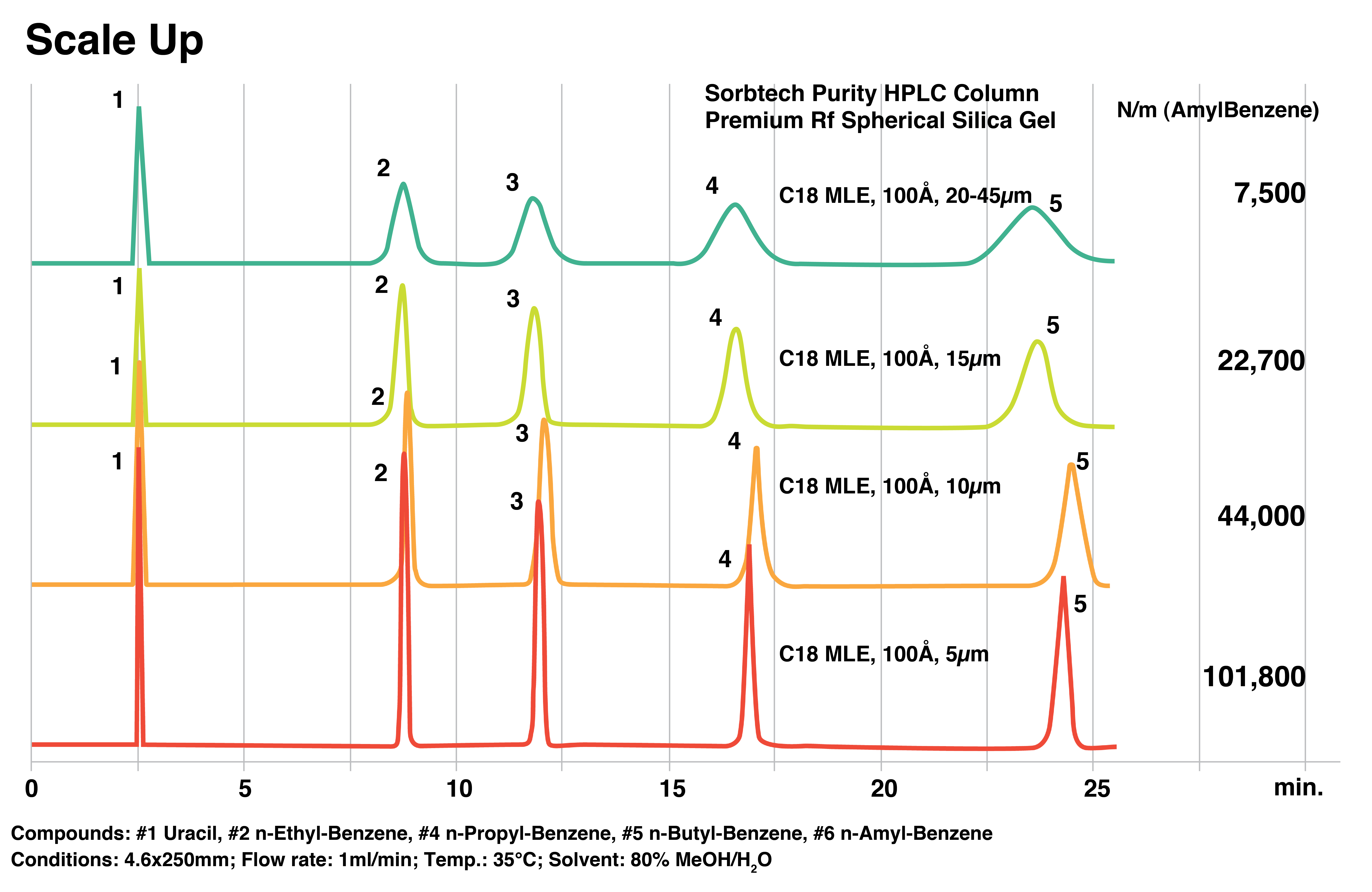
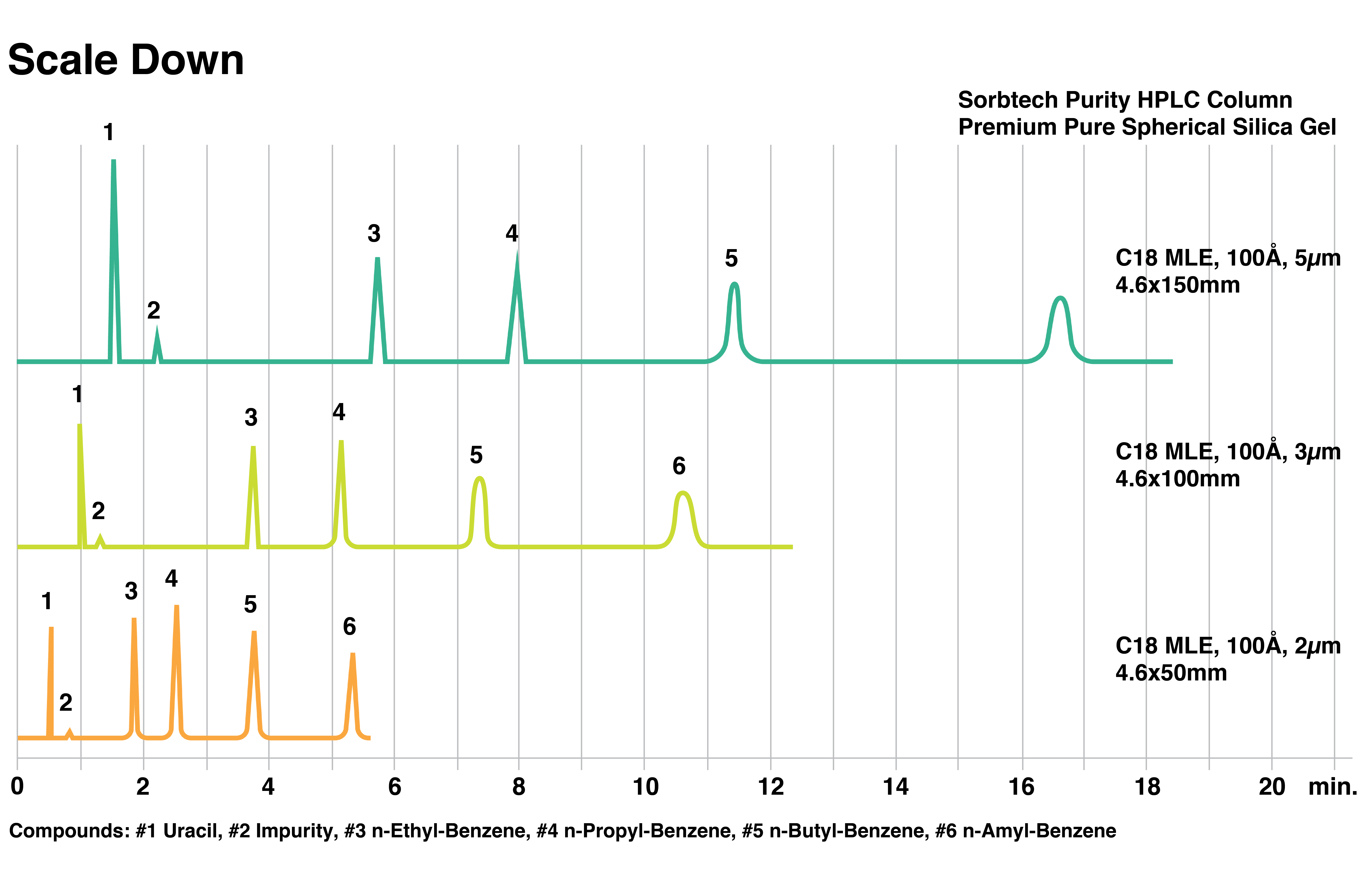
Scale up or Scale down with ease.
With Sorbtech, your laboratory research has a direct line to manufacturing by way of HPLC to Prep HPLC to Flash Column to Process Column methods, all without any change in base material or bonding technique.
For example, a researcher could use a Sorbtech Purity HPLC Column packed with 5µm, C18 spherical silica gel in the laboratory and then move to prep or process scale with 10µm, 15µm, 20-45µm, 40-75µm, or 75-200µm C18 spherical silica gel from the same supply source, thereby eliminating any variables due to the adsorbent media.