Introduction
Flash chromatography is a widely used technique in laboratories for the rapid separation and purification of compounds. One of the key concepts to understand and optimize in flash chromatography is the Plate Number. This article will explore what Plate Number is, its significance, and how it can be used to improve your flash chromatography results.
What is Plate Number
Plate Number is a mathematical construct used to measure the efficiency of a chromatographic column.
Origin of Plate Number
By the middle of the 20th century, all chemists knew about distillation, what it was, and how to use it. Distillation and crystallization were the primary methods of separation of mixtures at that time.
However, chromatography was the invention of a Russian Botanist 50 years earlier. Most chemists were German, English, French, Italian, and American. The result was that chromatography was not well accepted by most chemists.
A.J. Martin developed an explanation of separation by chromatography that related chromatography to distillation. For this work, Dr. Martin received the Nobel Prize in 1952 for this work.
Originally misnamed a “theoretical plate”, chemists now use the term plate number. Misnamed because there is nothing “theoretical” about it.
Significance of Theoretical Plates
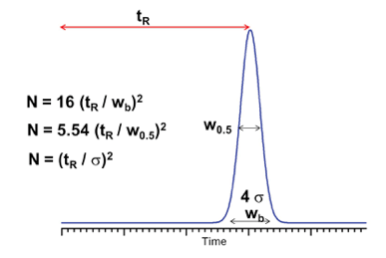
Where
N Plate Number
RT Retention Time (or volume)
W Width (or Peak Volume)
How to Calculate Plate Number from a Chromatogram
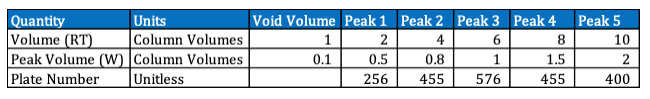
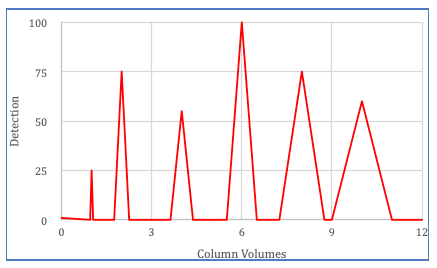
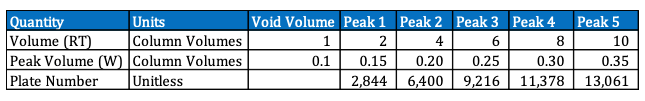
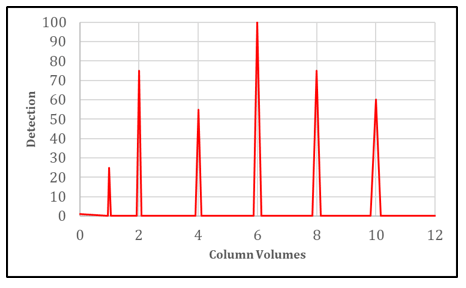
Each value of Plate Number represents a hypothetical stage where the solute equilibrates between the stationary phase and the mobile phase.
The more theoretical plates a column has, the more efficient it is at separating compounds.
A higher number of theoretical plates indicates better separation efficiency, meaning the compounds are more effectively separated as they pass through the column
Improving Flash Chromatography by Increasing Plate Number
Column Selection
- Columns with smaller particle sizes, longer lengths will generally have more theoretical plates, leading to better performance.
- The size of the column, including its diameter and length, must also be appropriate for the amount of sample you plan to purify.
- Smaller particle sizes will increase back pressure at flow rate.
Optimizing Flow Rate
- The flow rate of the mobile phase can significantly impact the number of theoretical plates.
- According to the Van Deemter equation, there is an optimal flow rate that minimizes band broadening and maximizes the number of theoretical plates.
- Exceeding fast flow rates beyond theoretical optimal will cause a deterioration in the efficiency and resolution of your separation. Hence the compromise between speed and efficiency/resolution must be addressed.
Temperature Control
- Maintaining a consistent temperature can reduce variations in the separation process, leading to a higher number of theoretical plates.
- Temperature fluctuations can cause changes in the viscosity of the mobile phase and the interaction between the analyte and the stationary phase.
Mobile Phase Composition
- The composition of the mobile phase can affect the efficiency of separation.
- Selecting the right solvent system is critical. A mixture of polar and non-polar solvents is highly recommended.
- You can determine the best solvent system by running thin layer chromatography (TLC) tests to find a solvent that gives an Rf value optimally at 0.3, although you can operate with solvent ratios within an Rf value between 0.1 and 0.4 for your target compound
Column Packing
- Properly packing the column to avoid voids and channels ensures a uniform flow of the mobile phase, which is crucial for achieving a high number of theoretical plates.
Pre-Column Treatment
- Pre-treat your sample with a short plug of silica to remove baseline impurities before loading it onto the main column, or alternatively incorporate a precolumn.
- Pre-columns are highly recommended in preparative purifications, especially with “Dirty” samples.
Practical Tips
Regular Maintenance
- Regularly clean and maintain your columns to prevent blockages and degradation, which can reduce the number of theoretical plates. Use a precolumn with challenging samples. Look for voids in the column bed.
- After each run, flush the column with a solvent of higher eluotropic strength than the mobile phase used.
- After flushing ensure to condition the column and prepare your column with your method’s starting solvent mixture.
Sample Preparation
- Ensure your samples are well-prepared and free from particulates that could clog the column and reduce efficiency.
Monitoring and Adjusting
- Continuously monitor the performance of your chromatography system and adjust parameters as needed to maintain optimal conditions. Pay particular attention to backpressure and separation efficiency to determine usable column life.
By understanding and optimizing the number of theoretical plates in your flash chromatography setup, you can achieve better separation and purification of compounds, leading to more accurate and reliable results.

Dr. Robert Kerr, Director of Research and Development
A native Texan, Dr. Kerr graduated from Austin College in Sherman, Texas, (B.A.), The University of Texas at Austin (M.A.), and L’Université Louis Pasteur in Strasbourg, France (D.E.A., D.Sc.). He assists chromatography customers with extensive knowledge in Pharmacy (Pharmacokinetics, Drug Synthesis, Formulations, Toxicology), Drug Submissions (Pharmacokinetics, Chemistry, and Toxicology), Natural Products Chemistry, Analytical Instrumentation (GC, GC-MS, HPLC, FTIR, NMR, ICP-MS, and others), and Organic Chemistry. Noted as a multi-disciplinary thinker with numerous patents and patent applications, Dr. Kerr can approach a problem from many different angles.


