Choosing Chiral GC Phases
In the following section, we provide guidance on selecting chiral GC phases. To do this, we studied the separation behavior of representative test substances from a variety of chemical groups using chiral MN columns. Specifically, we examined how each phase performed when separating spiro compounds, terpenes, aliphatic, cyclic and aromatic ketones, cyclic ethers, oxiranes, lactones, aromatic esters, aromatic amides, as well as cyclic, non-cyclic, and aromatic alcohols.
Chromatographic Conditions
To keep results consistent, all separations were run under identical conditions:
-
Column dimensions: 25 m length, 0.25 mm ID, 0.25 μm film
-
Carrier gas: Hydrogen, 0.6 bar, split flow 50 mL/min
-
Temperature program: Start at 60 °C, increase 2 °C/min until 200 °C
Evaluation Method
We evaluated the degree of separation using a points-based scale ranging from 0 to 10.
-
0 points: No separation of the enantiomer pair
-
1–5 points: Partial separation
-
6–10 points: Full baseline separation
This system accounted for both resolution and the overall level of difficulty.
First Step: Molecular Weight Influence
As a first step, we measured how the molecular weight of test substances affected the separation properties of HYDRODEX and LIPODEX phases. To compare performance, we used phases with different cyclodextrin ring sizes and observed how they handled enantiomeric separations under the same conditions.
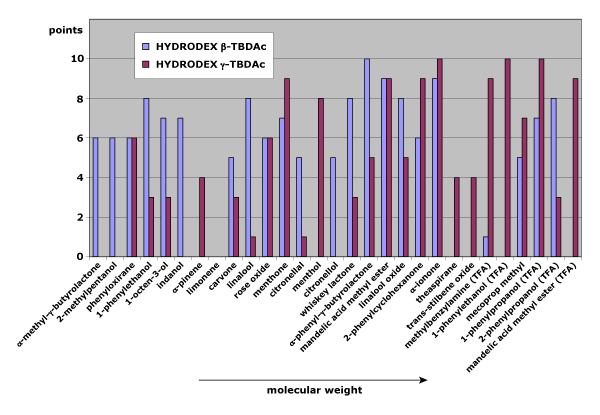
Fig. 1: Separation behavior on HYDRODEX β-TBDAc and γ-TBDAc as a function of the molecular weight of the analytes
Separation Quality with HYDRODEX Phases
Figure 1 compares separation quality (0–10 points) of test substances on HYDRODEX β-TBDAc (β-cyclodextrin phase) and HYDRODEX γ-TBDAc (γ-cyclodextrin phase, with a larger ring). Results show a clear trend: as analyte size increases, enantiomer pairs separate more effectively on the larger γ-cyclodextrin phase.
Separation Behavior with LIPODEX Phases
A similar trend appears with LIPODEX phases of varying cyclodextrin sizes. LIPODEX A (α-cyclodextrin), LIPODEX C (β-cyclodextrin), and LIPODEX E (γ-cyclodextrin) all display improved separation with larger analytes. However, the relatively bulky substituents in LIPODEX phases influence selectivity more strongly than in HYDRODEX phases, making the cyclodextrin ring effectively smaller. Even so, LIPODEX E maintains strong selectivity across the full molecular weight range (see Figure 2).
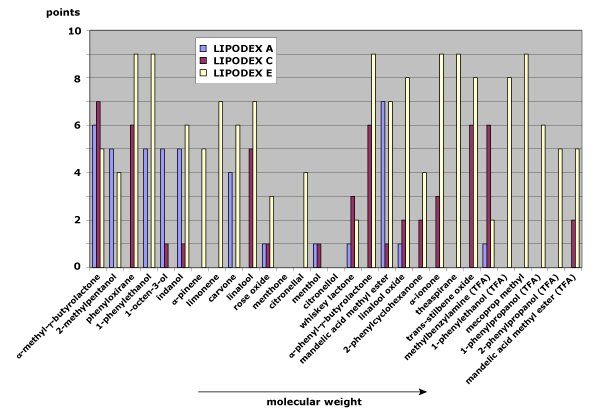
Fig. 2: Separation behavior on LIPODEX A, LIPODEX C, and LIPODEX E as a function of the molecular weight of the analytes
Effect of Cyclodextrin Substituents on Separation
Beyond the link between analyte size and cyclodextrin ring size, we also explored how cyclodextrin substituents interact with the functional groups of different analytes. These interactions reveal, how even small structural differences can shift separation efficiency.
Broad Selectivity of LIPODEX E
Among the tested phases, LIPODEX E once again shows the widest selectivity. It excels with esters (e.g., mandelic acid methyl ester, mecoprop methyl), cyclic ketones (e.g., α-phenyl-γ-butyrolactone, α-ionone), oxiranes (e.g., phenyloxirane, trans-stilbene oxide), and terpenes (e.g., α-pinene, limonene) (see Figure 3).
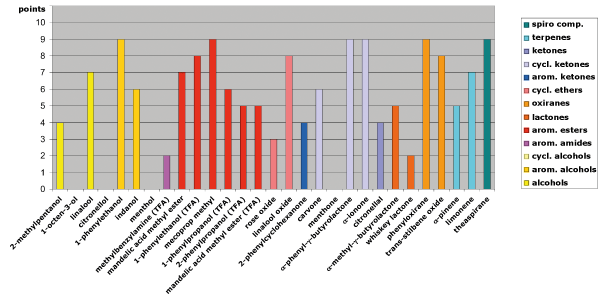
Fig. 3: Separation behavior on LIPODEX E in relation to the functional groups of the analytes
Separation Characteristics of LIPODEX Phases
LIPODEX A demonstrates strong selectivity for small alcohols. In contrast, LIPODEX B shows little to no accumulation across the studied compound groups under the given test conditions. LIPODEX C, however, performs well with cyclic compounds that contain polar groups, such as cyclic ketones and lactones.
LIPODEX D offers a broad range of selectivity, yet it does not show consistent accumulation within a group. In practice, this means that even if one compound in a group separates successfully, a structurally similar compound may not. Meanwhile, LIPODEX G has proven effective for separating underivatized alcohols, esters, and ketones.
Separation Characteristics of HYDRODEX Phases
The HYDRODEX series, also delivers strong results. In particular, HYDRODEX β-TBDAc provides excellent separation for various alcohols (e.g., 2-methylpentanol, linalool, 1-phenylethanol), ketones (e.g., α-phenyl-γ-butyrolactone), esters (e.g., mandelic acid methyl ester), and many polar-ring analytes (e.g., linalool oxide) (see Figure 4).
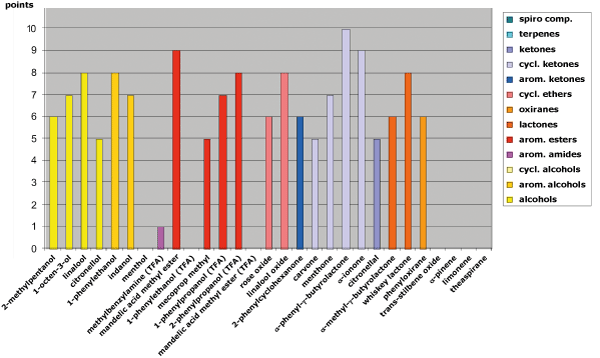
Fig. 4: Separation behavior on HYDRODEX β-TBDAc in relation to the functional groups of the analytes
Performance of HYDRODEX γ-TBDAc
The HYDRODEX γ-TBDAc phase, which carries the same modification (2,3-di-O-acetyl-6-O-t-butyldimethylsilyl) as HYDRODEX β-TBDAc but uses a larger cyclodextrin, delivers strong selectivity for esters, ketones, and amides. However, it provides only modest separation for low-molecular-weight alcohols. Interestingly, alcohols with a ring system near the chiral carbon atom can achieve good separations once they are esterified. Earlier phases such as HYDRODEX β-PM, β-3P, and β-6TBDM also achieved success, though only with selected compounds.
Limits of Existing Phases
Although later-developed phases like LIPODEX E and HYDRODEX β-TBDAc demonstrate broader applicability, they still cannot guarantee successful separation for every substance within a compound group.
Importance of New Phases
Because of these limitations, new phases such as HYDRODEX γ-DiMOM are especially valuable. They expand the range of enantioselective separations, making it possible to analyze substances that were previously difficult—or even impossible—to separate (e.g., pulegone – constituent of peppermint oil).
Many more applications of LIPODEX and HYDRODEX phases are available in the SORBTECH application data base.
Optimizing Chiral GC Separations
Once you select a chiral GC phase that shows promising separation properties and obtain initial or partial separations, the next step is method optimization.
Choosing the Right Carrier Gas
Hydrogen should be the preferred carrier gas because it delivers higher plate numbers at relatively low flow rates (40–80 cm/s). This makes it more effective than helium or nitrogen for chiral separations.
Managing Sample Load
Chiral GC columns can quickly show peak deformation when overloaded. To avoid this, reduce the injected sample amount. You can achieve this by combining high detector sensitivity with a large split flow, which minimizes overload while maintaining accuracy.
Balancing Temperature Control
Chiral separations are highly temperature-sensitive. Finding the optimal temperature usually requires compromise: lower temperatures improve resolution, while higher temperatures shorten analysis time. The goal is to strike a balance that ensures both efficiency and clarity.
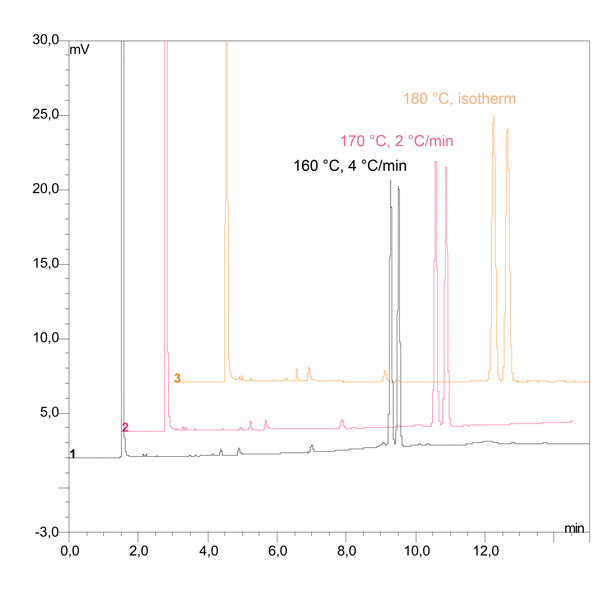
Fig. 5: Optimization of the temperature
Figure 5 shows the separation of 2-phenylhexanone at 180 °C under isothermal conditions and with different temperature programs. Results indicate that isothermal runs should be the first choice. However, if a temperature program is necessary, the heating rate should remain as low as possible—ideally between 2–4 °C per minute.
Conclusion
Chiral GC phases built on modified cyclodextrins make many enantiomer separations possible. Yet, predicting the exact selectivity of a chiral phase remains difficult.
Studies with LIPODEX and HYDRODEX columns highlight that both molecular size and comparisons with structurally similar substances can guide method development. These comparisons can help estimate outcomes, though they cannot guarantee success. Because of this, running a literature search before starting new separations often proves helpful. In addition, the Sorbtech application database and the Technical Support team at Sorbent Technologies provide valuable resources for method selection and troubleshooting.
References
[1] Gil-Av, B., Freibush, R., Charles-Sigler, R., Tetrahedr. Lett. (1966), 1009
[2] Frank, H. , Nicholson, G.J., Bayer, E. , J. Chromatogr. Sci. 15 (1977), 174
[3] Schurig, V., Schmalzing, D., Schleimer, M., Angew. Chem. Int. Ed. 30 (1991), 987
[4] Fischer, P., Aichholz, R., Bölz, U., Juza, M., Krimmer, S., Angew. Chem. Int. Ed. 29 (1990), 427
[5] König, W.A., Krebber, R., Mischnik, P., J. High Resolut. Chromatogr. Commun. 12 (1989), 732
[6] König, W.A., Enantioselective gas chromatography with modified cyclodextrins, Hüthig, Heidelberg, Germany (1992)
[7] Schurig, V., Nowotny, H.-P., J. Chromatogr. 441 (1988), 155
[8] Zhou, X.C., Yan, H., Chen, Y.Y., Wu, C.Y, Lu, X.R., J. Chromatogr. A 753 (1996), 269
[9] Pfeiffer, J., Schurig, V., J. Chromatogr. A 840 (1999), 14


