HPLC columns for the enantiomer separation
Molecules, which are like mirror images, are called “chiral” (from the Greek word for “hand”) because they are mirror-symmetrical – like hands. They cannot be separated with conventional HPLC columns, because the physical and chemical properties of enantiomers are identical except for geometric orientation. Chirality is of vital importance in biological processes. Thus, one of the most important drugs against stomach and intestinal ulcers esomeprazol – the (S)-enantiomer of omeprazol – shows enhanced activity in comparison with the racemate (a mixture of both enantiomers). This enhancement results from a slightly lower metabolism rate and thus from an increased bioavailability of the S-enantiomer.
Because of this difference in biological activity, regulatory authorities for pharmaceutical products require unambiguous separations of racemates. Thus, enantiomer separation by HPLC has become indispensable for the development and analysis of chiral drugs. It is also very important in biology and chemistry, especially in catalytic research, as well as for foodstuffs.
This article describes several concepts of HPLC columns for enantiomer separation with reference to the racemates to be separated. Finally, a flow chart is presented, which supports the sometimes demanding selection of a suitable column.
Enantiomer separation with ligand exchange phases
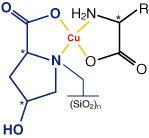
Ligand exchange phases for enantiomer separation in liquid chromatography were first described by Davankov et al. [1]. They are silica gels with covalently bonded copper(II)-chelate complexing agents as chiral selectors. The most important interaction between the enantiomers to be separated and the chiral selector is the formation of a transition metal complex with analyte and selector as ligands.
An L-hydroxyproline / Cu2+ complex serves as chiral selector for NUCLEOSIL®Chiral-1. Due to the asymmetry centers of hydroxyproline, diastereomeric complexes with the enantiomeric isomers of the analytes are formed which results in different retention times for the enantiomers due to the differences in the stability of the complexes.
A successful enantiomer separation can be expected, if the sample molecule contains two polar functional groups, which can form a chelate complex with copper ions, such as α-amino acids (e.g. phenylglycine, alanine, threonine etc.) and α-hydroxycarboxylic acids (e.g. lactic acid).
Aqueous CuSO4 solutions are used as eluents. By increasing the concentration of copper ions from 0.2 to 3.0 mmol/L the retention times can be reduced. A reduction of retention times, as well as an improvement of the peak symmetry, can also be reached by the addition of organic solvents such as acetonitrile or methanol. An increase in temperature – we recommend 60 °C – will lead to better separation efficiency with shorter retention times.
Chiral columns with π-donor/acceptor systems
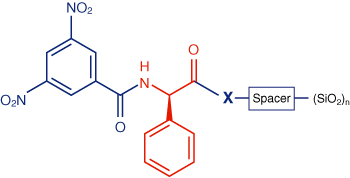
This type of chiral phase, which is used for the control of the optical purity of compounds under normal phase conditions, was first applied for the separation of racemic helicenes by Mikes et al. [3]. It is termed “brush type phase”, or also “Pirkle phase”, because the working group of Pirkle synthesized numerous versions of this type.
As a chiral selector for NUCLEOSIL® Chiral-2, dinitrobenzoyl-D-phenylglycine is covalently bonded to silica gel via a spacer, the optical antipode dinitrobenzoyl-L-phenylglycine is used for NUCLEOSIL® Chiral-3.
There are numerous applications for the separation of enantiomers and diastereomers, especially for the control of optical purity of herbicides (e.g. fenoprop methyl), insecticides (e.g. cypermethrin), or drugs (e.g. the anticoagulant phenprocoumon). For the determination of the optical purity of mecoprop methyl the less concentrated impurity can be eluted before the main peak by using NUCLEOSIL® Chiral-3, which contains the optical antipode of Chiral-2. Thus, a reliable assay is possible.
The separation mechanism could not yet be clarified in all details. But besides π-donor/acceptor interactions hydrogen bonds, dipole/dipole interactions, and steric effects determine a separation.
Nonpolar organic mobile phases, such as n-heptane, iso-octane with polar additives like tetrahydrofuran, alcohols, or chlorinated hydrocarbons are used for the application of these chiral columns. Small amounts of strong acids (e.g. trifluoroacetic acid) can considerably improve the separation of the enantiomers. The solubility of basic compounds can often be enhanced by derivatization (e.g. with benzoyl chloride or 3,5-dinitrobenzoyl chloride).