Have you ever developed a TLC plate and noticed how great your separation looked, distinctly separated spots that you’d be proud to show anyone who attempted a chromatographic separation? Next thing, you’re setting up a flash chromatography method, you’ve set your column, loaded your sample, and now you wait for your separated samples to elute.
Ah, the start of a peak appears on the screen, up, up, up it goes, apexes, then starts to fall back down, when suddenly, it starts going up again. The frustration you feel is no different than the frustration that many have felt in translating a method from TLC to flash chromatography.
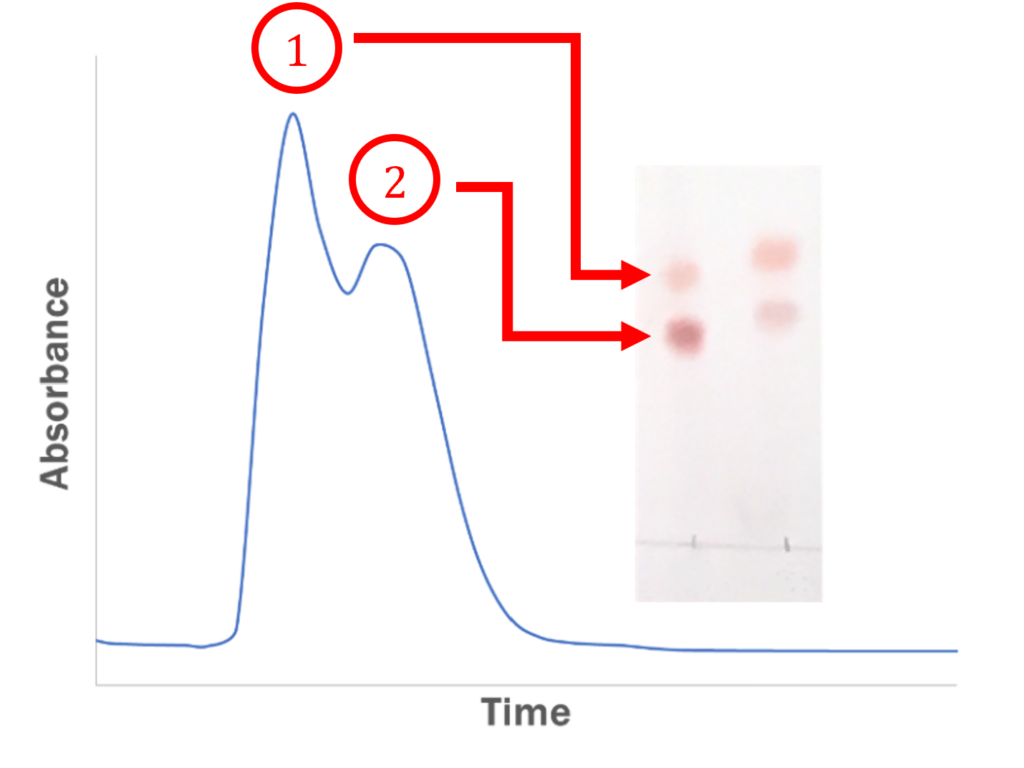
Wouldn’t it be great if your TLC plate separation could be transferred directly to a flash chromatography method. It is possible; however, the typical scale of a flash purification limits the ability to do so. Let’s get into some of the technical reasoning behind all of this.
Across the board the standard for TLC plates is typically a 5-15µm granular silica gel and the most common flash chromatography silica gel ranges from 40-63μm granular silica gel.
Question: Why are they different?
Answer: The driving force for solvent movement in TLC is capillarity. The smaller the particles, the better the capillarity and the faster the solvent movement. The driving force for solvent movement in Flash Chromatography is pressure. The smaller the particle, the higher the pressure required for solvent movement through the column.
For both TLC and Flash Chromatography, the smaller the particle, the higher the separation efficiency. With the larger particles, the diffusion of the sample is increased, which can lead to peak broadening. The peak broadening causes considerable difference in the desired separation, most notably the peaks overlapping as shown above.
This is often also due to the number of theoretical plates or the separating power of the silica. This phenomenon contributes to peak narrowing, which creates a more efficient separation between two or more samples.
Note: Sample Loading
What a lot of scientists also do not realize is the difference in loading on a TLC plate vs. loading a sample onto a flash column.
Very often your TLC plate will have reduced separation if your sample is still wet when development starts, this is very similar to how one may load a liquid sample onto a flash column. When a sample is dry loaded onto a column resolution is improved, just as on a TLC plate where the sample is usually dry.
So, why don’t we just use 5-15µm silica gel in a flash column?
Well, this is done, with HPLC. A lot of HPLC columns use 5µm or smaller, which leads to significantly higher resolution as well as separation efficiency. However, the pressure is also significantly higher and requires a steel column. Plastic (polypropylene) columns simply cannot withstand the higher pressures of particles less than 20 microns.
Steel columns are expensive and not disposable. This makes using smaller particles prohibitive for a flash column separation.
What we do see being used for flash chromatography instead of a 40-63µm silica is a 20-45µm silica gel. This improves the efficiency of the separation and gets much closer to a TLC like separation, while still maintaining a lower pressure system and allowing for it to be easier to pack.
While it may be difficult to reproduce the separation provided by a TLC plate, we can be very close to simulating it by modifying the size of the silica and/or by dry loading the sample onto the column.
Written by Robert Cotta


